Synthesis of Oxa-Bridged Medium-Sized Carbocyclic Rings via Prins Cyclization
By: Wang, Min-Shou; Wang, Zheng; Chen, Wen; Yang, Xiaodong; Zhang, Hongbin
ORGANIC LETTERS
Volume: 21
Issue: 6
Pages: 1881-1884
DOI: 10.1021/acs.orglett.9b00491
Published:MAR 15 2019
Document Type:Article
Abstract
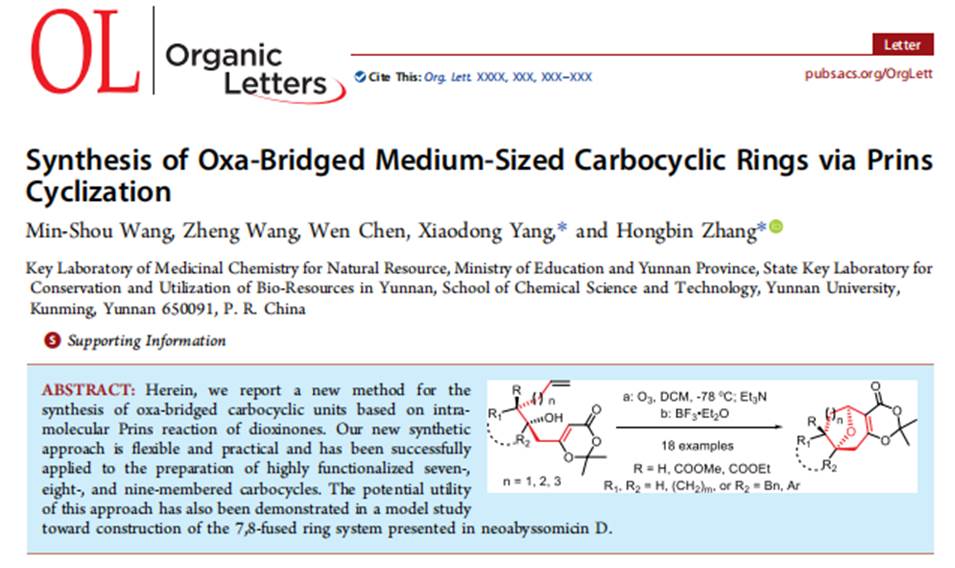
Herein, we report a new method for the synthesis of oxa-bridged carbocyclic units based on intra-molecular Prins reaction of dioxinones. Our new synthetic approach is flexible and practical and has been successfully applied to the preparation of highly functionalized seven-, eight-, and nine-membered carbocycles. The potential utility of this approach has also been demonstrated in a model study toward construction of the 7,8-fused ring system presented in neoabyssomicin D.
Key Words: ACID-PROMOTED 3+4; NEIGHBORING GROUP PARTICIPA-
TION; CATALYTIC ASYMMETRIC-SYNTHESIS; NATURAL-PRODUCT;
DICARBONYL ELECTROPHILES; CYCLOADDITION REACTION; ANNULA-
TION REACTIONS; DERIVATIVES; SESQUITERPENOIDS; COMBINATION
Author Information
Yang, XD; Zhang, HB (reprint author)
Yunnan Univ, Key Lab Med Chem Nat Resource, State Key Lab Conservat & Utilizat Bioresources Y, Minist Educ & Yunnan Prov,Sch Chem Sci & Technol, Kunming 650091, Yunnan, Peoples R China.
Addresses:
[1] Yunnan Univ, Key Lab Med Chem Nat Resource, State Key Lab Conservat & Utilizat Bioresources Y, Minist Educ & Yunnan Prov,Sch Chem Sci & Technol, Kunming 650091, Yunnan, Peoples R China
全文链接: https://sci-hub.tw/10.1021/acs.orglett.9b00491