Three-Component Cascade Reaction of 1,1-Enediamines, N,N‑Dimethylformamide Dimethyl Acetal, and 1,3-Dicarbonyl Compounds: Selective Synthesis of Diverse 2‑Aminopyridine Derivatives
BY: Quan-Xing Zi, Sheng-Jiao Yan,* Chang-Long Yang, Kun Li, and Jun Lin*
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education and Yunnan Province, School of Chemical Science and Technology, Yunnan University , Kunming 650091 , P. R. China
ACS Omega,2019,4(2), pp 2863–2873
DOI:10.1021/acsomega.8b03284
Publication Date (Web): February 7, 2019
Abstract
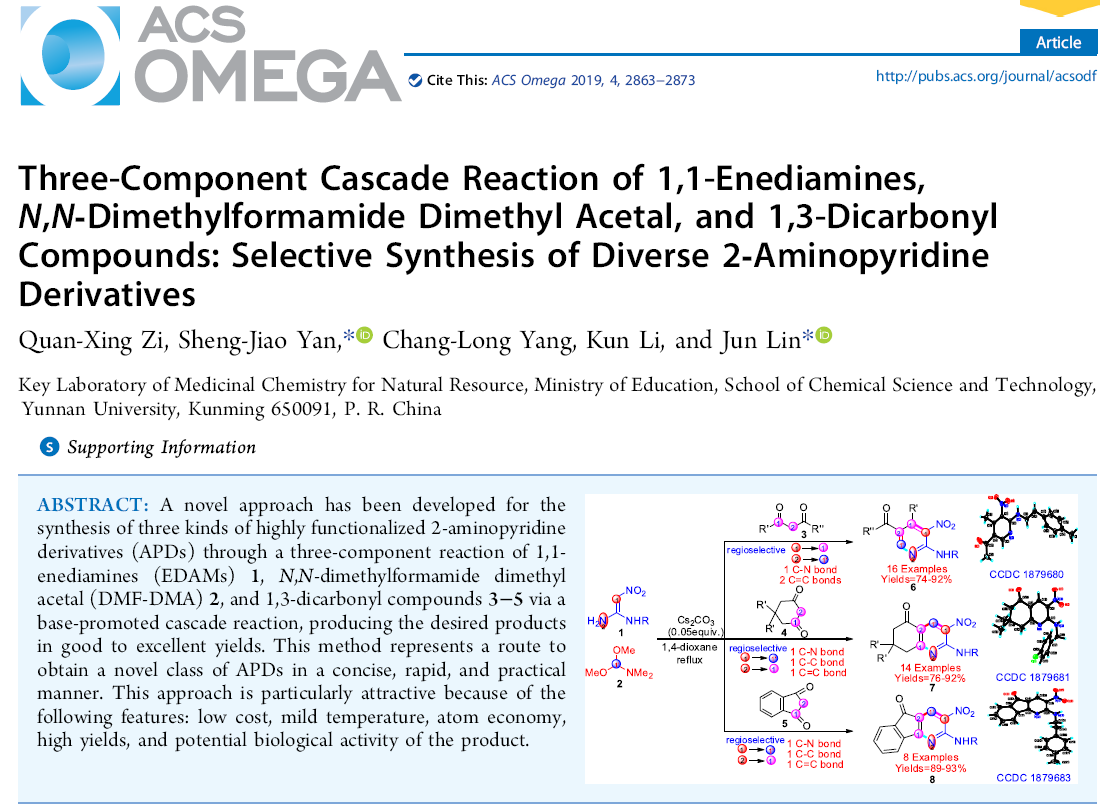
A novel approach has been developed for the synthesis of three kinds of highly functionalized 2-aminopyridine derivatives (APDs) through a three-component reaction of 1,1- enediamines (EDAMs) 1, N,N-dimethylformamide dimethylacetal (DMF-DMA) 2, and 1,3-dicarbonyl compounds 3−5 via a base-promoted cascade reaction, producing the desired products in good to excellent yields. This method represents a route to obtain a novel class of APDs in a concise, rapid, and practical manner. This approach is particularly attractive because of the following features: low cost, mild temperature, atom economy,high yields, and potential biological activity of the product.
全文链接:https://pubsdc3.acs.org/doi/abs/10.1021%2Facsomega.8b03284