By:Ma, Xiurong;Wang, Guixi; Zhou, Qian; Liu, Yanxiong; He, Runying; Xie, Zuoxun; Shi, Yonggang; Cao, Qiue; Zheng, Liyan
Journal of Medicinal Chemistry
DOI:https://doi.org/10.1021/acs.jmedchem.5c02006
Published:2025-11-13
Abstract
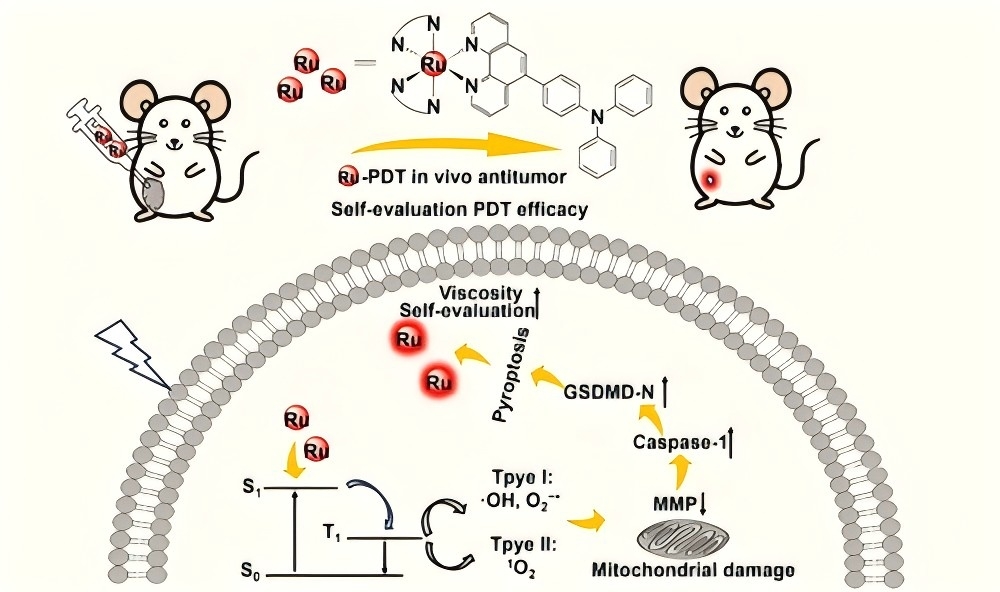
The development of a photosensitizer (PS) integrating real-time therapeutic monitoring capability represents a critical advancement in precision photodynamic therapy (PDT). Herein, three ruthenium(II)-triphenylamine photosensitizers (Ru1-3) were designed and synthesized. Detailed antitumor mechanism investigations revealed that Ru1 and Ru3 activated caspase-1, induced gasdermin D (GSDMD) cleavage and mitochondrial membrane potential (MMP) depolarization, and triggered cell pyroptosis. An increase in the luminescence intensity of Ru1 and Ru3 was observed during cell pyroptosis, indicating a significant augmentation of cytoplasmic viscosity during pyroptosis. Moreover, two-photon excitation experiments verified that they possessed photocytotoxicity in 3D multicellular tumor spheroids (MCTSs), and a similar luminescence amplification correlated with pyroptosis progression was observed. In vivo studies confirmed that Ru3 exhibits superior PDT efficacy and provides real-time feedback on treatment response via its self-reporting luminescence characteristics. This work provides new insights into the design and implementation of multifunctional photosensitizers for tumor cell pyroptosis with real-time therapeutic monitoring and two-photon imaging.