Coupling of Acyl Radical Precursors with 2-Azaallyl Anions for the Synthesis of α-Amino Ketones
By: Haitao Yang; Yu Pan; Canli Zhang; Chen Chen; HaoqinTang; HongbinZhang; Guogang Deng; Xiaodong Yang
Organic Letters
DIO: https://doi.org/10.1021/acs.orglett.5c01295
Published:2025-05-01
Abstract
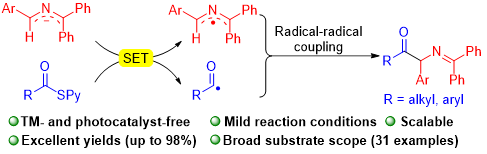
A novel transition-metal-free radical coupling of 2-azaallyl anions for the synthesis of α-amino ketones has been developed. Easily accessible thioesters and 2-azaallyl anions undergo single electron transfer (SET) to generate acyl radicals, which participate in intermolecular radical coupling to generate α-amino ketones with good functional group tolerance and yields (31 examples, up to 98% yield). A telescoped gram-scale synthesis and derivatization of the product illustrate the potential synthetic utility of this method. Radical trapping and radical clock experiments support the proposed radical coupling pathway between the generated acyl radical and the 2-azaallyl radical.